Most biotechnology clean room facility have get high value production line and equipment (Machinery).
And also complex production process, strict requirement for class standard for the clean room facility, as well as the request the aseptic working place.
Potential biological hazards occur in the production process, mainly (infection risk, dead bacteria or dead cells and components or metabolism to human and other biological toxicity, sensitization and other biological reactions, product toxicity, sensitization and other biological reactions, environmental effects. )
Clean room area:
A clean room (area) in which dust particles and microbial contamination in the environment need to be controlled, and its building structure, equipment and use have the function of preventing the introduction, generation and retention of pollutants in the area.
Airlock area:
An isolated space with two or more doors between two or more rooms, such as those with different cleanliness levels. The purpose of the air lock is to control the flow of air when the person or material is in and out. There are personnel air lock and material air lock.
The basic characteristic of purification chamber of biotechnology:
It is necessary to take dust particles and microorganisms as environmental control objects.
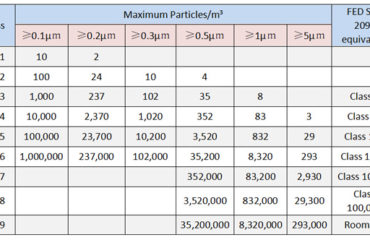
The cleanliness of pharmaceutical production workshop is divided into four levels Class100, Class1,000, Class10,000 and Class100,000 The same as ISO 5,ISO 6, ISO 7,and ISO 8.
The temperature and Humidity for a clean room: The temperature range is between 18℃ to 26℃, the relative humidity is between 45% to 65%.
Biotechnology clean room plant pollution control: pollution source control, dissemination process control, cross-contamination control.
The key technology of medicine in purification clean room is mainly to control dust and microorganism, as a pollutant, microorganism is the most important part of environmental control in purification clean room. The equipment in the clean area of the pharmaceutical factory building and the pollutants accumulated in the pipeline can directly pollute the medicine, but it does not affect the cleanliness test, so we say: GMP need air purification technology, and air purification technology does not represent GMP. Cleanliness grade is not applicable to characterize the physical, chemical, radiological, and life of suspended particles. Not familiar with the pharmaceutical production process and process, do not understand the causes of pollution and the site of the accumulation of pollutants, do not master the methods and evaluation criteria for the removal of pollutants.