Purification principle of GMP pharmaceutical clean room plant
Monday, 25 October 2021
●Adopt the new three-level filter of the air filtration system, fresh air tertiary filter (initial, middle, sub-efficient filter), intermediate filter, and end efficient filter. Make the airflow organization more scientific and reasonable (can increase the life of meter cooler, medium efficiency filter and high efficiency filter) to change the medium effect filter bag screw fixation
Main technical parameters of the clean room for GMP pharmaceutical plant
Tuesday, 19 October 2021
●Indoor air changes: Class 100,000 10-15 times/hour; Class 10,000 15-25 times/hour; Class 1,000 50-52 times/hour; ●Average wind speed of Class 100 operating point section 0.25-0.35m/s. Indoor noise: ≤ 65dB (A); ●Indoor pressure difference: high clean room area for adjacent low clean room area ≥ 5Pa, to non-clean room area ≥ 10Pa; ●Indoor temperature: Winter :16℃±
Latest technology for a standard GMP clean room of pharmaceutical facility
Thursday, 23 September 2021
A new “absolute blocking technology” has been certified by the FDA and the European Medical Regulatory Commission. At present, there are more than 200 laboratories and more than 100 advanced pharmaceutical enterprises have begun to apply, the technology between the protection area between non-protection area, break through the traditional clean room technology, logistics buffer step
Static pressure difference in a turnkey modular clean room
Wednesday, 15 September 2021
In order to avoid the cleanliness of the turnkey modular clean room by the pollution near the non-clean room, the appropriate static pressure difference shall be maintained between the clean room and the general room, and the static pressure difference shall not be less than 5Pa.The difference of pressure from the outdoor is not less
Brief introduction the noise standard for a dusty free clean room
Monday, 06 September 2021
Regarding the dusty free clean room while under working, in addition to testing and controlling the cleanliness, temperature and humidity, the noise standards are also required to be controlled. Human and purification analysis of clean room noise standards and clean room noise control methods. The exhaust system noise has a great impact on the clean
Reasonable layout for standard modular clean room project
Friday, 03 September 2021
The layout of clean room project should pay attention to the simple plane shape, clear functional partition, and reasonable distribution of pipeline concealed space, flexibility of production process and equipment renewal, and safety of fire evacuation. The plane combination form of clean room project often adopts adjacent, block, enclosure and other combinations. Organize the space
The microbiology laboratory of the clean room
Wednesday, 25 August 2021
The basic idea of laboratory design is economical and practical. The purification requirement level is class 10000. The laboratory design has preparations for experiments such as greater, secondary, wind shower and buffer. The principle of separating flow and logistics is adopted to reduce experimental pollution and ensure safety. The layout is compact and reasonable, meeting
There are 4 different air supply and air return plans for a clean room project
Monday, 16 August 2021
Here are the advantages and disadvantages for the different plans for a general clean room working area. ●Air supply and air return all from the ceiling Suitable for the class standard: ISO7, ISO8 Advantages: Simple and low cost Disadvantages: Poor airflow resistance is difficult to effectively control dust ●Air supply from the ceiling & air
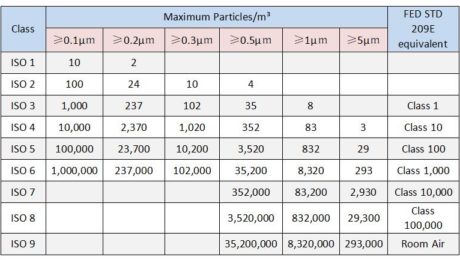
How to classification the different class standard to a clean room facility in pharmaceutical industry
Wednesday, 11 August 2021
Generally, there are 4 class standard for the production of sterile drugs in a pharmaceutical facility. ●ISO 5/ Class 100: High risk operating areas such as filling areas, areas where rubber plugs are placed and open packaging containers in direct contact with sterile preparations and areas where sterile assembly or connection operations shall have the